It's estimated that 1 in 8 American women will develop some form of breast cancer over the course of their lifetime.
After decades of rigorous research, the U.S. Food and Drug Administration (FDA) approved the first drug aimed to treat women with advanced breast cancers, particularly the ones caused by BRCA 1 and BRCA 2 gene mutations.
This form of breast cancer became quite notable after Academy Award-winning actress Angelina Jolie revealed she underwent a double mastectomy after testing positive for BRCA 1.
Jolie removed her breasts after being told she's at high risk for developing breast cancer, and later removed her ovaries and Fallopian tubes after a second health scare.
Dubbed the "Jolie gene," these flawed genes increase a woman's risk of developing breast tumors from 12% to 90%.
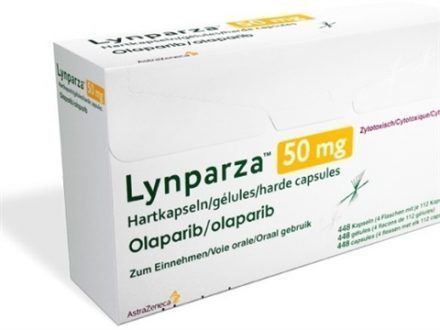
On Friday, the highly anticipated drug, known as Lynparza, can finally be used on patients, and it has everyone wondering how it's going to work and how much it will cost.
Lynparza can be used on patients with BRCA gene mutations who have undergone chemotherapy.
"We are delighted that the FDA has approved [Lynparza] for advanced breast cancer in women who have inherited BRCA1 or BRCA2 mutations," said Professor Andrew Tutt, director of the Breast Cancer Now Research Cancer in the UK, which played a vital role in developing the drug.
"This is excellent news for women with this uncommon but important genetic form of breast cancer, many of whom took part in the clinical trials," he added.
The medication prevents cancer cells from fixing problems in their DNA, which then causes those cells to die or decreases the growth of the tumor.
This breakthrough is pivotal in breast cancer treatment because it makes treatment and prevention less invasive, and also more manageable.
Also, side effects are less severe than for chemotherapy, but can include serious effects like blood and bone marrow cancers.
"The trial measured the length of time the tumors did not have significant growth after treatment [progression-free survival]," the FDA explained. "The median progression-free survival for patients taking Lynparza was 7 months compared to 4.2 months for patients taking chemotherapy only."
The pharmaceutical company, AstraZeneca, said the drug will cost around $13,886 per month without insurance.