Last month, we reported on a group of recalls affecting generic liquid infant ibuprofen products on sale at several national chains including Walmart and CVS.
The products, which were sold under several store brand names, were manufactured by Tris Pharma of New Jersey and packaged in 0.5 ounce bottles.
The bottles were recalled because they potentially contain a higher dosage of ibuprofen than they are meant to, which could be unsafe.
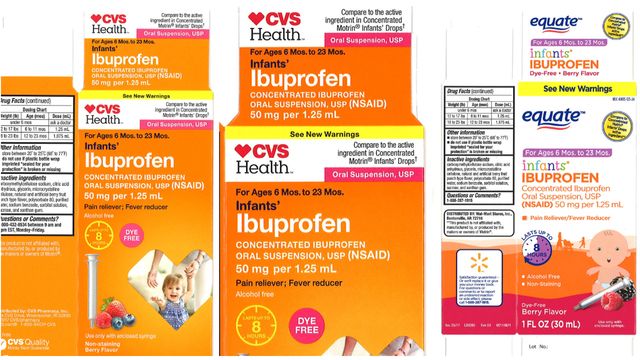
Major chains including Walmart, CVS and Family Dollar offered the medicines under brand names such as CVS Health, Family Wellness, and Equate.
In a statement about the recall, Tris Pharma warned that infants could suffer a "permanent NSAID-associated [nonsteroidal anti-inflammatory drug] renal [kidney] injury" from taking the unsafe dosage.
Less serious symptoms from taking the tainted medication could include nausea, vomiting, diarrhea, stomach pain, tinnitus (ringing ears), and internal bleeding. (So far, no customers have reported any adverse effects caused by the recalled medicines.)
This week, the FDA issued an expanded recall for even more oral ibuprofen products, including larger 1 ounce bottles.
Here's a complete list of the lots now being recalled, including the ones from last month:
CVS Health: Infants' Ibuprofen Concentrated Oral Suspension, USP, 50 mg per 1.25 mL, in 0.5 oz. bottle
CVS Pharmacy
Lot No.: 4718
NDC: 59779-925-23
EXPIRATION: 12/19
Equate: Infants' Ibuprofen Concentrated Oral Suspension, USP, 50 mg per 1.25 mL, in 1.0 oz. bottle
Wal-Mart Stores Inc.
Lot No. 00717005A
NDC: 49035-125-24
EXPIRATION: 02/19
CVS Health: Infants' Ibuprofen Concentrated Oral Suspension, USP, 50 mg per 1.25 mL, in 1.0 oz. bottle
CVS Pharmacy
Lot No. 00717006A
NDC: 59779-925-24 (Labeled as: 50428-1252-4)
EXPIRATION: 02/19
Equate: Ibuprofen Oral Suspension Drops, USP, 50 mg per 1.25 ml, in 0.5 oz. bottle
Wal-Mart Stores Inc.
Lot No. 00717009A
NDC: 49035-125-23
EXPIRATION: 02/19
Equate: Ibuprofen Oral Suspension Drops, USP, 50 mg per 1.25 ml, in 0.5 oz. bottle
Wal-Mart Stores Inc.
Lot No. 00717015A
NDC: 49035-125-23
EXPIRATION: 04/19
Equate: Ibuprofen Oral Suspension Drops, USP, 50 mg per 1.25 ml, in 0.5 oz. bottle
Wal-Mart Stores Inc.
Lot No. 00717024A
NDC: 49035-125-23
EXPIRATION: 08/19
Equate: CVS Health: Ibuprofen Oral Suspension Drops, USP, 50 mg per 1.25 ml, in 0.5 oz. bottle
CVS Pharmacy
NDC: 59779-925-23
EXPIRATION: 08/19
Family Wellness: Ibuprofen Oral Suspension Drops, USP, 50 mg per 1.25 ml, in 0.5 oz. bottle
Family Dollar Services Inc.
NDC: 55319-250-23
EXPIRATION: 08/19
Customers who purchased the products were warned to throw them away immediately, or return them for a refund.
You can report a reaction to a medication using the FDA's MedWatch form.
You can contact Tris Pharma's customer service at (732) 940-0358 with questions regarding the recall.
Read the full recall notice from the FDA here. Read Tris Pharma's updated recall notice here.
[H/T: ABC 13]

