When you consider how painful, aggravating, and seriously annoying migraines can be, it's a wonder we still don't know how to cure them.
As many as 10 million Americans get migraines, mainly women in their thirties and beyond.
If you live with migraines, you know how easy it can be to lose a day or even a week to the debilitating pain.
There's good news for migraine patients now that a drug to prevent migraines has been approved by the FDA.
But there are some important facts you should know about Aimovig.
A first-of-its-kind drug
Up until now, patients have had to treat migraines and cluster headaches with run-of-the-mill headache medicine, or odd treatments like Botox and epilepsy medications.
While these treatments can (sometimes) give relief from migraine pain, they don't prevent migraines from happening.
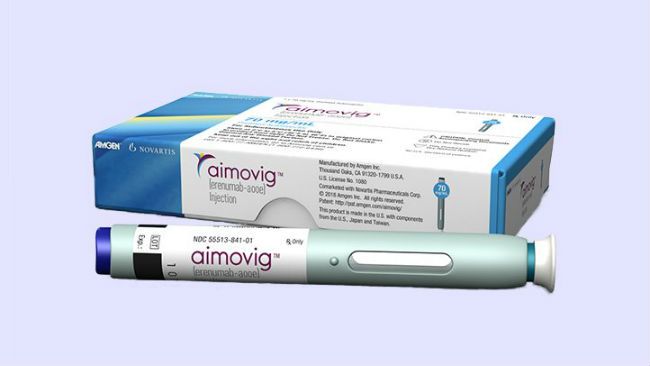
Aimovig (called erenumab generically) is the first FDA approved drug that prevents migraines before they happen.

It's developed by California's Amgen Inc. and the Swiss drugmaker Novartis AG.
Patients inject themselves once a month with a special pen device, and the drug is meant to stop migraines before they start.
Promising results
To test Aimovig, researchers split migraine patients into two groups.
The first group were injected with placebo "dummy" shots, while the second group took Aimovig.
On average, the first group noticed an average of two less migraine days a month. Patients on Aimovig noticed an average of four less migraine days.
Some patients who took Aimovig even described being completely cured, according to Amgen's research department.
But patient in both groups noticed side effects, including colds and respiratory infections.
Aimovig seems to cause less daily side effects than other migraine drugs, which can cause drowsiness and trouble thinking.
But more testing needs to be done to learn if Aimovig has long-term effects, and whether it's safe for pregnant women.
Costs and concerns
If you're dying to try Aimovig, you'd better have health insurace.
Amgen says that the drug is expected to cost $6,900 annually (or $575 a month) without insurance.
There are also concerns about whether Aimovig is a smart choice for patients with heart conditions.

The drug works by using special antibodies to block receptors for a substance called CGRP, which spikes in your brain's bloodstream during a migraine.
The trouble is that CGRP helps relax arteries. If the substance is blocked, it interrupts blood flow and slow a patient's heart rate during an emergency, like a heart attack.
If you're concerned that Aimovig is not for you, you won't have to wait long for more options.
New migraine shots and even pills are scheduled to hit the market in the next few years.
Living with migraines? We have more information to help you cope:
- 15 surprising things that can trigger a migraine.
- 7 ways to naturally kick a migraine before it ruins your day.
- 10 products that will naturally help anyone who suffers from migraines.
[H/T: Time, Science News, Forbes]