A drug manufacturer has recalled its liquid baby ibuprofen products after discovering the dose contained in the medicine could be higher than the label warns.
New Jersey's Tris Pharma issued a voluntary recall of three lots of "Infants' Ibuprofen Concentrated Oral Suspension, USP (NSAID)" in 0.5 ounce bottles.
You can view the lot numbers of all recalled products here.
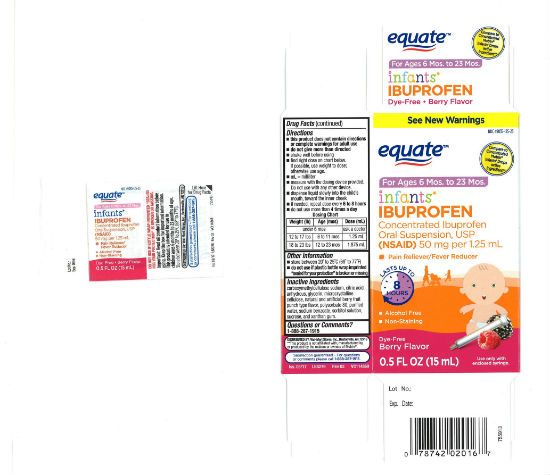
The company warned that the recalled bottles could contain more ibuprofen than they should, and are not safe to use.
It's a serious recall, because the affected drugs were sold at major chains including Walmart, CVS, and Family Dollar under several different store-brand names, including CVS Health, Family Wellness, and Equate.
"There is a remote possibility that infants, who may be more susceptible to a higher potency level of drug, and therefore may be more vulnerable to permanent NSAID-associated renal injury," Tris Pharma said in a statement.
"Adverse effects that may be experienced are nausea, vomiting, epigastric pain, or more rarely, diarrhea. Tinnitus, headache. and gastrointestinal bleeding are also possible adverse effects."
While Tris Pharma says no one has reported a reaction, so far, they are recalling all affected products out of an abundance of caution.
If you purchased one of the affected products you should throw them out immediately.
If your child experiences any symptoms that could be related to this product, contact their doctor right away.
You can also report a reaction to the medication using the FDA's MedWatch form.
You can contact Tris Pharma's customer service at (732) 940-0358 with questions regarding the recall.


